Bopyrid Isopods in the Aquarium

The Parasitic Relationship between Bopyrid Isopods & their Hosts: with Special Reference to the Parasitism of Ornamental Crustacea by Bopyrid Species
If you have ever asked the question whats that strange lump on the side of my peppermint shrimp? chances are you have had a shrimp with a parasitic isopod from the Bopyridae family. This family of isopods acts similarly to the common leech, latching onto its host and slowly sucking the hosts blood. Once fully developed, bopyrid are easy to spot as the presence of the large female induces the carapace of the host to grow around the parasite, creating an abnormal discolored bulge. These ectoparasites are a relatively common site to the staff here at Quality Marine, as crustaceans are always in high demand by aquarists, we handle them by the thousands. Through our experience we have found these parasites most common in shrimp of the Lysmata genus, with the Atlantic peppermint shrimp, Lysmata sp. (multiple species identified), having the highest frequency of occurrence, followed by Lysmata amboinensis. We hope this article will help every day aquarists, store owners and other industry professionals learn a little more about these mysterious and extraordinarily fascinating creatures.

The peppermint shrimp, Lysmata boggessi, with isopods attached. We believe that the parasitic isopods found under their carapace were of the Parabopyrella mortenseni species
Classification
- Kingdom Animalia (animals)
- Phylum Arthropoda (arthropods)
- Class Malacostraca
- Order Isopoda (isopods, pillbugs)
- Suborder Epicaridea
- Family Bopyridae
- Suborder Epicaridea
- Order Isopoda (isopods, pillbugs)
- Class Malacostraca
- Phylum Arthropoda (arthropods)
The Order Isopoda consists of approximately 5,000 species. Within this Order is the Suborder Epicaridea which is derived from the Latin words "Epi" meaning "on" and "carid" which means shrimp. Aptly named, the isopods in this Suborder are most commonly but not exclusively found on shrimp. With the exception of several species, adult bopyrids generally occur in the branchial chamber (gills) or on the abdomen of the host, depending on the subfamily. All bopyrids are holoparasites (dependent on their host for nourishment) of free-living decapod crustaceans, such as shrimp and crabs. Bopyrid isopods make up the largest family of isopods with over 500 different species identified worldwide, they are also considered to be the most biologically advanced family within the order.
The Life Cycle of the Bopyrid
Females brood hundreds of small eggs in their marsupium, also known as the oostegial brood pouch. It is in this brood pouch where eggs embryonate and later hatch into a free swimming epicaridium larvae. In this initial larval stage they have six pairs of clawed pereopods which will eventually be used for latching onto their intermediate hosts. After hatching, the larvae have approximately two weeks to search for an intermediate host, if they do not find one within that time they will starve to death (Lester 2005). The intermediate host is most commonly a type of calanoid copepod, which once found will be used to serve as the nourishment and staging point for more transformations by the bopyrid. To feed itself, the bopyrid pierces through the exoskeleton of the copepod and starts to feed by on the haemolymph, a combination of blood and interstitial fluid, of the copepod. After feeding for a few days the bopyrid molts into the microniscus stage. During this stage, the bopyrid will continue to feed on the copepod for several weeks and will rapidly enlarge up to 10 times its original size through multiple molts. Once the microniscus stage is complete the bopyrid morphs into the cryptoniscus larval stage, a process done without molting but rather by expanding folds in the cuticle (Anderson and Dale 1981). The cryptoniscus is sexually undifferentiated (Reinhard, 1949), with no designation of it being a male or female. It is at this free swimming stage when the Bopyrid detaches from the intermediate host copepod and searches for a suitable decapod to become its final host.
If an uninfected definitive host is found, the bopyrid will latch on and climb into its final position, normally on the abdomen or within the branchial chamber. In branchial parasites, the bopyrid attaches ventrally to the host"s branchiostegite (within the gill chamber). The isopod will latch onto the decapod using its pereopods (claws) and commences feeding off of the decapod host much like was done with the preceding intermediate copepod host. At the same time the bopyrid parasite will develop into a female and begins to grow significantly larger. The now female parasite also becomes greatly modified in its final transformation, losing its appendages, as it no longer has the need to swim, and transforms into its final stage as a bopyridium. At full adult size, the bopyrid parasites are easily identified on their host, as they cause a characteristic bulge on the side of their carapace as their presence forces the carapace to grow over the parasite. At a quick glance the parasite doesnt look like an isopod, but rather looks like a large white lump on the side of a shrimp
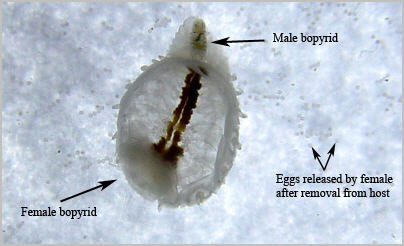
A close up of the underside of a female bopyrid, surrounded by eggs which were released immediately upon removal from the host. The male can be seen at top within the females pleopods
A simplified flow of the life cycle can be seen as follows:
Adult -> Egg -> Epicaridium -> Microniscus -> Cryptoniscidium -> Adult (bopyridium)
Adult female bopyrids attract cryptonisci, presumably using pheromones. The cryptonisci attracted to the female will find her, attach directly to her, and then commence developing into a dwarf male. In observing the 10 samples taken at our facility, all females removed from the shrimp Lysmata boggessi (a species of Atlantic peppermint shrimp) had a single male attached to her pleon. There were no incidences of multiple males being found and no incidences of females without a male. In the study, males from two different female bopyrid were physically removed and placed near each other. Within a minute the two males were observed attacking one-another, with one clearly being dominant. This observation leads to the assumption that intruding males on an already established pair are fought by the resident male, with the winner remaining with the established female. Perhaps this explains why multiple males were not found near the female parasites

A close up of the male within the females pleopods
Throughout the course of their adult life as a pair, the dwarf males will remain a fraction of the size of the full grown females. The males are not parasites of the decapod and do not normally come in contact with the hosts tissue. It is presumed that they may even be a hyperparasite of the female as it is not yet known how the males derive their nutrition. They will spend most of their time attached to the pleopods of the female and after the female molts, the male will climb inside of the brood pouch of the female, most likely for insemination and within several hours, a new batch of eggs is created (Cash and Bauer 1993). The reproductive potential of a mature female bopyrid is extraordinary as it can produce a hundreds of eggs at one time in her marsupium. There are a series of flaps on the ventral (underside) of the Bopyrid to keep the eggs in place within the marsupium (Goater 1996). In many species the release of the epicaridea (larvae) and the molt of the female bopyrid are synchronized with the molt of the host decapod and will occur a few days prior (Lester 2005).
Having the ability to change sexes into a male or female, depending on whether or not the host decapod has already been parasitized, leads to the assumption that bopyrids are sequential hermaphrodites. In sequential hermaphrodites the gonads of the organism can fully convert from one sex to another. The gonads also possess the genetic information to produce both male and female reproductive organs, but only the dominant gene is expressed at any give time (Ho 2002). In the unlikely event that the female dies during the prior to the host decapod, it is presumed that the male can then switch sexes and become the female parasite.
The Effects on Bopyrids on their Hosts
The effect of bopyrids varies from species to species, most species do not significantly harm their host despite their very obtrusive appearance however some species have been reported to cause massive trauma to their hosts. Females feed on host haemolymph by piercing a blood sinus usually on the inside wall of the gill cover or branchiostegite. Studies have shown that during the initial stages of parasitism the bopyrid can take up to 25% of the shrimps haemolymph in one day (Lester 2005). This rate is not believed to be continuous and should lessen as the bopyrid reaches full adult size. A separate study of the Probopyrus isopon on Palaemonetes (glass shrimp) showed that the bopyrid sequesters approximately 10% of the hosts energy intake and reduces egg production by 50% (Anderson 1977). As a result parasitized shrimps have less energy for basic needs such as capturing food and migrating to breeding sites (Somers and Kirkwood 1991).
There have been a variety of different studies done on the other effects of the bopyrid isopods on their hosts. One study monitored the effect of an abdominal bopyrid isopod on the protandric simultaneous hermaphrodite shrimp Lysmata seticaudata (the Mediterranean equivalent to the peppermint shrimp). The results showed that parasitized shrimp were not able to produce embryos, but still were capable of successfully fertilizing the eggs of unparasitized shrimp (Calado et al. 2004). This shows that the bopyrid somehow castrated the female gonads of the shrimp while the male gonads still functioned normally. It is not yet known how the bopyrid castrates the shrimp, one of several theories suggests that the nutritional drain affecting the energy flow could impair the production of energy needing vitellogenic oocytes (Anderson 1977). This essentially means that the parasites drainage of its hosts energy is too great for the full development of the female sex organs to occur.
A separate study showed that parasitized shrimp exhibited a reduced tolerance to stress due to environmental changes in salinity and temperature (Moles and Pela 1984). This is one factor to consider with aquarium species, as there are often many more fluctuations in closed systems than in nature. The bopyrid can also cause a deformation of gills on the host decapods due to pressure atrophy (Schuldt and Rodrigues-Capitulo 1987). This can greatly hinder the uptake of oxygen and make the host even more susceptible to stress caused by environmental changes.

Species of Ornamental Crustacea that may be Affected by Bopyrids
Bopyrid Isopods are occasionally found on a wide variety of decapods including fiddler crabs, porcelain crabs, and shrimp of the Lysmata genus. The parasites are more frequently seen in the several available varieties of peppermint shrimp from the Western Atlantic and Gulf of Mexico, including Lysmata wurdemanni, L. rathbunae, L. ankeri, L. boggessi, L. pederseni, and L. bahia. Similar peppermint shrimp specimens from other waters are also known to occasionally be infected with Epicaridean isopods, such examples are: the close relatives of the Atlantic species of peppermint shrimp, Lysmata seticaudata (Mediterranean Sea) and Lysmata californica (Eastern Pacific Ocean). Additionally, the Scarlet Skunk Cleaner Shrimp, Lysmata amboinensis, is also known to frequently be parasitized by bopyrid isopods. From our experience cleaner shrimp collected from Sri Lanka have a much lower parasitized incident rate than those collected from Indonesia and Philippines. Another frequently parasitized species is the porcelain crab, Petrolisthes sp.
In the peppermint shrimp of various species that we import from the Gulf-Atlantic, we have a fluctuation of rates of shrimp that are parasitized. A poor shipment would yield a rate of approximately 25 out of 3000 which equals 1 in 120 or an .83% chance of a shrimp being infected. This is a very low incidence rate and most parasite infected shrimp are culled out of the batch, therefore there is an even lower probability that an aquarist will ever encounter a bopyrid.
Implications of Bopyrid in Aquaria
As already mentioned the bopyrid can cause slight to major effects on their hosts. In the most common cases in aquaria, such as those reported on shrimp of the Lysmata genus, the isopods do not seem to cause too much damage. However, due to their reported increase in sensitivity to salinity fluctuations when parasitized, the aquarist should always do their best to keep consistent levels.
In the event that a shrimp or crab with a bopyrid is added to the aquarium, the aquarist need not panic. As mentioned above, the epicardium larvae require an intermediate host copepod, typically a specific calanoid copepod species, in order to survive and continue to the next stages in their lifecycle. In marine aquariums, the most common type of copepod is a harpacticoid, a dertrivore which tends to cling to rocks. According to Dr. Adelaide Rhodes (personal communication, November 6, 2006) calanoid copepods, characterized by their large, frilly antennae are uncommon due to the typical environments of saltwater aquaria. Besides requiring a diet of live phytoplankton, they generally do not fare well with the high currents associated with these tanks as they like to stay up in the water column where they are easily pulled into pump intakes and destroyed. Without these vital intermediate hosts present, there is little chance that any bopyrid larvae could successfully mature to adults and be able to become a parasite of other shrimp.

A common cleaner shrimp, Lysmata amboinensis, infected by a bopyrid parasite. (photo by Duane Wilde
Removal of the Parasite in the Gill Cavity from Host Decapods
If aquarists are still worried about having a living bopyrid in their system, it is a fairly easy process to remove the parasites from their hosts. While we have not tested the long-term survivability after the procedure, we feel that if done carefully the isopod can be removed without causing any permanent damage of the host.
Once the host has been captured we recommend that it is placed in a medium bowl of tank water. It should be large and deep enough for you to work on the host while keeping it submerged. The specimen should be handled very lightly throughout the process and care should be taken not to damage any of the body parts or subject the host to excessive stress.
We recommend the use of a very fine pair of tweezers in the removal of the parasite. First gently hold the host upside down underwater, providing access to the gill cavity. Gently slide the closed tweezer head beneath the gill plate just below the isopod, carefully making sure that the body of the shrimp is not punctured. The isopods (plural, as the dwarf male is usually attached to the female) do not have a particularly firm grasp on the shrimp and if grabbed lightly with the tweezers they should slip out relatively effortlessly. At this point it is best to remove the isopods promptly from the water as the female will begin to immediately disperse many epicardium larvae and eggs if they are available in the brood pouch. We believe this to be an automatic mechanism in the female to attempt to spread her seed in the event she is somehow removed. It is also recommended that the parasite be removed under water because the void in the carapace from the isopods being removed can leave a pocket where an air bubble can easily get caught. This air bubble if left could provide difficulties during the next molt and is best removed. If the procedure is done out of water, be sure to remove any residual air bubbles collected under the carapace. This can be done simply by submerging the host and lightly pulling back the gill chamber until the air bubble floats out of the chamber. The shrimp can then be put back in the main aquarium and the water were the procedure was done should then be discarded. The deformity in the carapace will most likely subside with subsequent molts, although some deformities have been reported to remain for the entire life of the host, typically in gonochoric female hosts (Lester 2005).
Bibliography:
Anderson, G. & Dale, W. (1981). Probopyrus pandalicola (Packard) (Isopoda, Epicaridea): Morphology and development of larvae culture. Crustaceana 41: 143-161.
Anderson, G. (1977). The effects of parasitism on energy flow through laboratory shrimp populations. Mar. Biol. 42, 239 251.
Boyko, C.B. (2004) The Bopyridae (Crustacea: Isopoda) of Taiwan. Zoological Studies 43(4): (pp. 677-703) Taipei, Taiwan 115, R.O.C.
Calado, R., Bartilotti, C., and Narciso, L. (2004). Short report on the effect of a parasitic isopod on the reproductive performance of a shrimp. Biotechnology Technical Information Service. Retrieved November 6, 2006 from http://www.aseanbiotechnology.info/Abstract/21017...
Cash, C.E., Bauer, R.T. (1993). Adaptations of the branchial ectoparasite Probopyrus pandalicola (Isopoda: Bopyridae) for survival and reproduction related to ecdysis of the host, Palaemonetes pugio (Caridea: Palaemonidae). Journal of crustacean biology. Vol. 13, no. 1, pp. 111-124.
Chadwick, V.J. (1989) Prevalence, Size and Fecundity of the Parasitic Isopod Argeia pugettensis on its Host Shrimp Crangon francisorum. American Midland Naturalist, Vol. 121, No. 1 (Jan., 1989), pp. 68-77
Goater, T. (1996). Parasitic Isopoda. Retrieved November 12, 2006 from http://web.mala.bc.ca/goatert/parasite/paraiso.ht...
Ho, L. (2002). Hermaphroditism: A Tale of Two Sexes. Retrieved November 14, 2006 from http://www.reefscapes.net/articles/articles/2002/...
Integrated Taxonomic Information System - ITIS * North America. (2006 May 11). Taxonomic Information for Parabopyrella mortenseni. Retrieved November 6, 2006, from http://www.cbif.gc.ca/pls/itisca/next?v_tsn=54599...
Lester, R.J.G. (2005). Crustacean Parasites: Isopoda (isopods). In K. Rohde (Ed.), Marine Parasitology (pp. 141-144). Collingwood, Victoria. Australia: CSIRO Publishing.
Markham, J. C. (1985). A review of the bopyrid isopods infesting caridean shrimps in the northwestern Atlantic Ocean, with special reference to those collected during the Hourglass cruises in the Gulf of Mexico. Fla. Dept. Nat. Resour., Bureau Mar. Res., St. Petersburg. 156 p.
Moles, A. and Pella, J.J.( 1984). Effects of Parasitism and Temperature on Salinity Tolerance of the Kelp Shrimp Eualus suckleyi. Transactions of the American Fisheries Society, 113:354359
Reinhard, E.G (1949). Experiments on the Determination and Differentiation of Sex in the Bopyrid Stegophryxus-Hyptis Thomson. Biological Bulletin, 96(1):17-31
Rhyne, A.L. and Lin, J.A. (2006). Western Atlantic Peppermint Shrimp Complex: Redescription of Lysmata wurdemanni, Description of Four New Species, and Remarks on Lysmata rathbunae(Crustacea: Decapoda: Hippolytidae). Bulletin of Marine Science, Vol. 79, No. 1.
Schimek, R.L. (2002). Pills, Parasites, and Predators; Isopods in the Reef Aquarium. Reefkeeping Magazine. Retrieved November 4, 2006 from www.reefkeeping.com/issues/2002-05/rs/index.php
Schuldt M, Rodrigues-Capitulo A (1987). Biological and pathological aspects of parasitism in the branchial chamber of Palaemonetes argentinus (Crustacea: Decapoda) by infestation with Probopyrus oviformis (Crustacea: Isopoda). J. Invert. Pathol. 45: 139-46.
Shields, J. (2006). Epicaridea: The parasitic isopods of Crustacea. Retreived November 8, 2006, from http://www.vims.edu/~jeff/isopod.htm
Somers, I. F., and G. P. Kirkwood (1991). Population ecology of the grooved tiger prawn, Penaeus semisulcatus, in the north-western Gulf of Carpentaria, Australia: growth, movement, age structure and infestation by the bopyrid parasite Epipenaeon ingens. Aust. J. Mar. Freshwater Res. 42:349467.